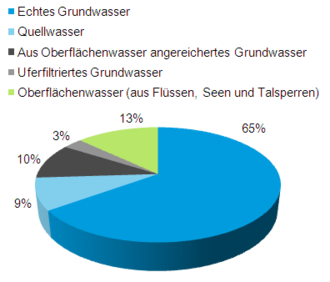
Wasseranteile an der Trinkwasserversorgung in Deutschland nach [2]
[1] Höll, Karl: Wasser. Berlin [u.a.]: 8., völlig neu bearb. Aufl. Aufl. de Gruyter, 2002.
[2] Merkl, Gerhard: Technik der Wasserversorgung. München: Oldenbourg Industrieverlag, 2008.
Depending on the properties of the aquifer and the length of time it has been underground, groundwater contains different amounts of chemical and biological ingredients as well as the composition of the rock in question. Reduced groundwater often occurs, which dissolves increased amounts of iron and manganese and therefore does not meet the requirements of the Drinking Water Ordinance.


Calcified submersible pump
A high-quality and sustainably safe water supply from groundwater therefore usually also includes water treatment to remove iron and manganese. The technology used for this and its efficiency determine the economic viability and environmental friendliness of the entire water supply and therefore play a crucial role for water suppliers.
DIN 2000[1] defines requirements for drinking water, among other things, with the guiding principle “Drinking water should be appetising and stimulate enjoyment. It must be colourless, clear, cool and have a perfect smell and taste. […] It must at least meet the legal requirements”.

The legal basis for the assessment of drinking water quality in Germany is the Drinking Water Ordinance (TrinkwV 2001[2] as implementation of Directive 98/83/EC[3]). It states:
“Drinking water must be of such a quality that its consumption or use does not pose a risk of harm to human health, particularly through pathogens. It must be pure and fit for consumption. This requirement is considered to be met if at least the generally accepted rules of technology are observed during water treatment and water distribution and the drinking water meets the requirements of Sections 5 to 7.”
Limit values according to the Drinking Water Ordinance
Iron and manganese are the two most common heavy metals in the earth’s crust, often occur together in minerals and are often both present in elevated concentrations in groundwater. The geogenic concentrations are usually well below the range relevant to the health of an adult. The Drinking Water Ordinance sets significantly lower limit values for sensory and technical reasons[4].
[1] DIN 2000: Zentrale Trinkwasserversorgung – Leitsätze und Anforderungen an Trinkwasser – Planung, Bau, Betrieb und Instandhaltung der Versorgungsanlagen; Technische Regel des DVGW
[2] TrinkwV 2001: Trinkwasserverordnung in der Fassung der Bekanntmachung vom 2. August 2013 (BGBl. I S. 2977)
[3] Richtlinie 98/83/EG des Rates: über die Qualität von Wasser für den menschlichen Gebrauch.
[4] Höll, Karl: Wasser. Berlin [u.a.]: 8., völlig neu bearb. Aufl. Aufl. de Gruyter, 2002.
Extract from the Drinking Water Ordinance[2]
§ 7 Indikatorparameter und Anhang 3
| Parameter | limit |
|---|---|
| Ammonium: | 0,5 mg/l |
| Iron: | 0,2 mg/l |
| Manganese: | 0,05 mg/l |
These low limit values are sensible because iron concentrations > 0.5 mg/l result in directly perceptible changes (brown discoloration of the water when exposed to air, change in the taste of the water). From 0.2 mg/l, discoloration and deposits can also occur on surfaces that come into contact with water, which limits both usability (from the customer’s perspective) and technical operational safety (from the supplier’s perspective → ochre).
In addition, deposits of iron oxide hydrates in pipes can form the basis for undesirable microorganisms[4]. To avoid this, the drinking water supplied by the waterworks should not contain more than 0.05 mg/l of iron[5]. The DVGW even recommends iron concentrations ≤0.02 mg/l and manganese concentrations ≤0.01 mg/l.[6] In reduced groundwater, in addition to iron and manganese, ammonium is often also found in increased concentrations. A limit value is also set for this in the Drinking Water Ordinance for general hygiene reasons. In general, compliance with these limits is mandatory for the supply of drinking water, but most water suppliers place significantly higher demands on their own drinking water.
This also corresponds to the minimization requirement required in the Drinking Water Ordinance and DIN 2000: “Concentrations of chemical substances that can contaminate drinking water or adversely affect its quality should be kept as low as is possible with reasonable effort in accordance with the generally accepted rules of technology.”[2]
This implies that water suppliers must choose a treatment technology that is as efficient and economical as possible. In the case of increased concentrations of iron, manganese or ammonium, underground iron removal and manganese removal (UEE) is recommended according to DVGW worksheet W 223[6].
[1] DIN 2000: Zentrale Trinkwasserversorgung – Leitsätze und Anforderungen an Trinkwasser – Planung, Bau, Betrieb und Instandhaltung der Versorgungsanlagen; Technische Regel des DVGW
[2]TrinkwV 2001: Trinkwasserverordnung in der Fassung der Bekanntmachung vom 2. August 2013 (BGBl. I S. 2977)
[3] Richtlinie 98/83/EG des Rates: über die Qualität von Wasser für den menschlichen Gebrauch.
[4] Höll, Karl: Wasser. Berlin [u.a.]: 8., völlig neu bearb. Aufl. Aufl. de Gruyter, 2002.
[5] Grohmann, Andreas ; Hässelbarth, U. ; Schwerdtfeger, W.: Die Trinkwasserverordnung. Berlin: 4., neu bearb. Aufl. Aufl. E. Schmidt, 2003.
[6] DVGW: Arbeitsblatt W 223-1. 2005.
Various processes between chemical and biological components of the groundwater and the composition of the soil lead to well aging or calcification in the course of well operation.
This refers to the reduction in well performance, primarily due to the deposition of reaction products in the well structure or in the adjacent rock and the resulting reduction in the cavity volume.[1]

Partially ochered wound wire filter
As soon as the water inlet areas into the well structure are reduced, the flow rate drops or the lowering of the operating water level increases while the withdrawal volume remains the same.
The main causes are:
- chemical and biological ochre formation
- sintering
- slime formation
- silting
While silting can result from errors in well construction, the other causes depend primarily on the quality of the raw water and the operation of the well.[2]
Clogging as the most common form of well aging
„The oxidation of dissolved, divalent iron and manganese and the resulting precipitation of insoluble iron(III) or manganese(IV) compounds is the most common cause of well aging.“ [3]
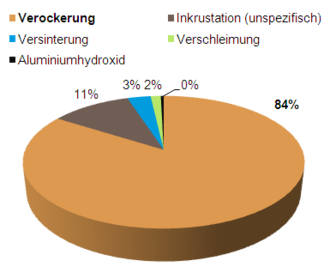
Ageing processes of wells in Germany according to a DVGW survey[5]
A 2009 DVGW survey found that two thirds of all waterworks operators experience well aging, which is usually detected by loss of yield or directly by camera inspection. This aging requires the regeneration and rehabilitation of wells, which most waterworks operators carry out at intervals of 1 to 5 years.[1]
A previous DVGW survey showed that the cause is almost always iron oxide. Over 80% of this is iron oxide, the rest is manganese oxide.[1]
Chemical and biological ochre
Chemical ochre is caused by the oxidation of divalent species when dissolved iron (Fe2+) or manganese ions (Mn2+) come into contact with dissolved oxygen. The reaction rate depends strongly on the pH value, but increases significantly due to the autocatalytic effect of oxides that have already formed. Since the oxidation of iron and manganese requires different redox potentials, either rust-brown iron ochre or black manganese ochre occurs, but rarely both. Where both species are present in the groundwater, only the iron is oxidized initially.

At lower redox potentials, biological ochre deposits occur in the majority of cases. The oxidation is catalyzed by sessile bacteria (e.g. by “iron bacteria”: Gallionella ferruginea, Leptothrix ochracea and discophorus). These bacteria require a regular supply of nutrients from water flowing past. They are harmless to drinking water hygiene, but in addition to the biomass they form large amounts of oxides (as well as hydroxides and oxide hydrates).
The deposits are generally subject to an aging process in which initially highly water-containing amorphous oxides change into denser crystalline forms, which are then more stable and difficult to dissolve. Iron-related incrustations usually occur in the form of ferrihydrite (Fe5HO8 x 4 H2O) and goethite (FeOOH)..[1]
Conclusion:
In summary, the ochre formation of wells is based on the following factors:
- Dissolved iron or manganese ions (according to specialist literature at iron concentrations > 0.2 mg/l[8])
- A positive redox potential / dissolved oxygen
- A pH value > 5
- An increased flow rate
- Iron and manganese-storing bacteria

Clogging of an underwater pump impeller
These factors are often present in well structures. If the performance drops by 10% to 20% due to ochre, an advanced stage of aging has already been reached and “regenerating it requires a high level of technical effort and therefore high costs.”[9]
As a preventive or remedial measure, attempts are often made to positively influence the oxygen level and the entry speed in the filter area through structural measures. But the most efficient method of preventing ochre is the use of underground iron and manganese removal.
This efficiently removes iron and manganese from the water before it enters the well structure.
[1] Houben, Georg ; Treskatis, Christoph: Regenerierung und Sanierung von Brunnen. München: Oldenbourg Industrieverl, 2003.
[2] Tholen, Michael: Arbeitshilfen für den Brunnenbauer. 2. Aufl. 2012.
[3] Wiacek, Hella: Brunnenmonitoring zur optimalen Brunnennutzung und -pflege. Johannes Gutenberg-Universität Johannes Gutenberg-Universität in Mainz, Fachbereich Chemie, Pharmazie und Geowissenschaften, 2005
[4] Orlikowski, D ; Dauchy, L ; Schwarzmüller, H: Ergebnisse der bundesweiten DVGW-Umfrage zur Instandhaltung von Brunnen. 2009.
[5] Houben, Georg ; Treskatis, Christoph: Regenerierung und Sanierung von Brunnen. München: Oldenbourg Industrieverl, 2003.
[6] Niehues, B.: DVGW-Umfrage ”Brunnenregenerierung”. 1999.
[7] Houben, Georg ; Treskatis, Christoph: Regenerierung und Sanierung von Brunnen. München: Oldenbourg Industrieverl, 2003.
[8] Mutschmann, Johann ; Stimmelmayr, Fritz: Taschenbuch der Wasserversorgung. Wiesbaden: 14. Aufl. Friedr. Vieweg & Sohn Verlag, 2007.
[9] DVGW: Arbeitsblatt W 130, 2007.
The solution: Underground iron and manganese removal (UEE)
The underground iron and manganese removal of groundwater (process water treatment) (UEE) with FERMANOX® activates a natural treatment process in the aquifer before extraction. With little effort, iron and manganese concentrations at drinking water level can be generated there. This effectively prevents all discoloration and ochre formation. At the same time, the process is particularly sustainable because
- no filter material is required,
- no waste water or waste is produced,
- energy requirements are particularly low and
- the service life of the boreholes is increased.

FERMANOX®-Plant for underground iron and manganese removal
The process: Underground iron and manganese removal with FERMANOX
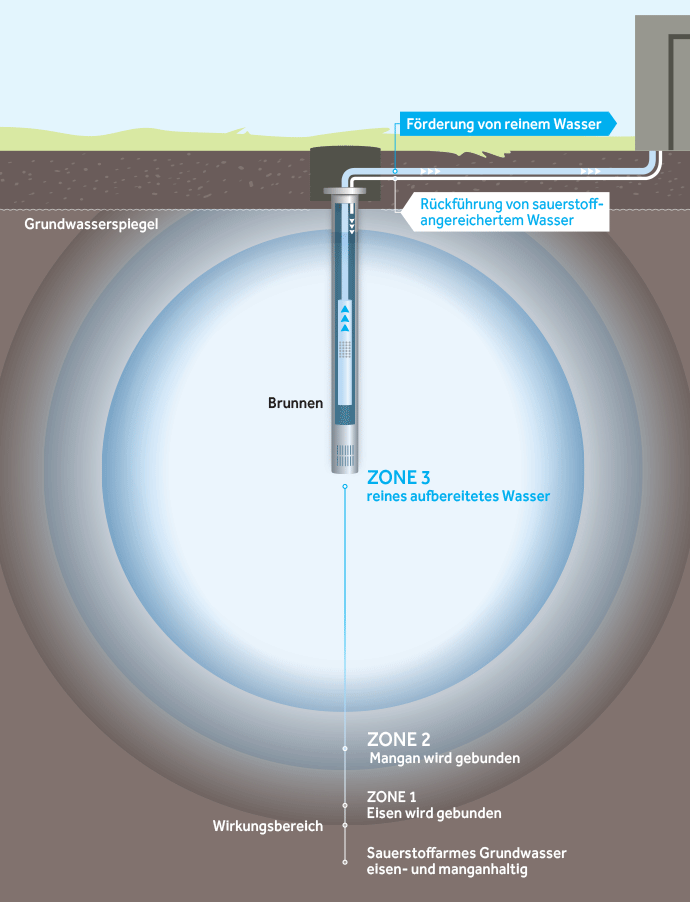
Oxidation zones in the aquifer when using an UWE
The basic idea behind underground deferrification and demanganization is the introduction of water enriched with atmospheric oxygen into the aquifer. Even small amounts of oxygen activate an effective water treatment process there, because a reaction space with an increased redox potential is created around the well.
The substances that were dissolved in the aquifer due to reduced conditions are returned to the solid state by oxidation and are then permanently fixed again in the same aquifer at another location. This achieves efficient deferrification and demanganization of the groundwater and also nitrification of ammonium, a reduction in easily oxidizable organic substances and a reduction in arsenic.
Continuous underground water treatment is based on the operation of at least two wells that work alternately as extraction or infiltration wells. The size of the reaction space around each well is determined by the amount of infiltration and the proportion of active pore volume in the aquifer. It requires an individual design for each well, which is based primarily on the raw water quality and the required treatment capacity.
History
The underground iron and manganese removal of groundwater has been used on a large scale since the 1970s, and since the 1980s in a variety of small and medium-sized plants for drinking and industrial water treatment, primarily in Germany, the Netherlands and Scandinavia, in various designs. Winkelnkemper GmbH has been manufacturing FERMANOX® water treatment systems since 1984 and is now the market leader in the field of underground iron and manganese removal, with over 10,000 compact systems installed.
In contrast to above-ground filter systems, underground iron and manganese removal (UEE) uses the aquifer itself in the area close to the well as a reaction space and for the retention of the reaction products. Within the reaction space, the oxygen introduced activates the complex treatment mechanisms. The chemical-physical processes are supported by an autocatalytic effect of the already determined oxides. Microbiological processes also play an important role.
The oxidation of the dissolved iron can be expressed, for example, using the following molecular formula:
2 Fe2+ + ½ O2 + (x + 2) H2O → (Fe2O3 * x H2O) + 4 H+[1]
In conventional above-ground filters, aeration produces mainly amorphous, water-rich iron(III) hydroxide (Fe(OH)3) and manganese oxide hydrate (MnOOH), and thus large quantities of sludge that must be backwashed and disposed of.
Underground, the primary reaction product iron(III) hydroxide is converted into crystalline iron(III) oxide hydrate and manganese(III) oxide hydrate into manganese dioxide (MnO2, “manganese dioxide”). The products form fine coatings on the soil grain surface with high density and correspondingly low volume.[1]
Iron and manganese oxide hydrates deposited on the soil grain lead to a high adsorption capacity for iron and manganese ions in the extraction phase. The oxygen introduced during the infiltration phase reaches the iron and manganese ions adsorbed on the grain surface as it flows through the aquifer and oxidizes them to poorly soluble compounds. Part of the oxygen is adsorbed and is still available for oxidation at the start of extraction.
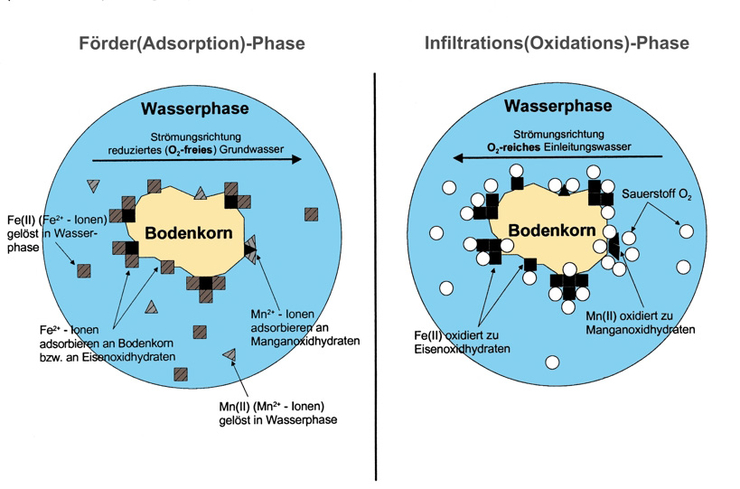
Adsorption-oxidation model – chemical-physical processes in the reaction space of the UEE (courtesy of Prof. Dr.-Ing. U. Rott).
In addition to iron and manganese removal, the introduced oxygen causes other positive reactions in the aquifer:
- Nitrification of ammonium and nitrite[2]: Ammonium and/or nitrite in groundwater are nitrified and thus significantly reduced. The conversion always takes place bacteriologically and, in the case of ammonium, always in two stages. 2 NH4+ + 3 O2→ 2 NO2– + 2 H2O + 4 H+ (+ Energy) by Nitrosomonas and Nitrosocystis 2 NO2– + O2→ 2 NO3– (+ Energy) by Nitrobacter and NitrosocystisThe resulting biomass is small and usually of no importance.
- Hydrogen sulphide and the unpleasant smell associated with it are eliminated by oxidation.
- Heavy metals such as arsenic, nickel etc. can be reduced. Since these substances are stored in the iron oxidation products, it is necessary that the groundwater has a minimum iron content. [3]
- When oxygen is introduced into the groundwater, methane oxidizes before iron in the aquifer, resulting in a relatively high biomass formation when oxygen demand is high; a special operating mode is therefore required for groundwater containing methane.
By appropriately designing an underground water treatment system, all of the above reactions take place in the aquifer outside the well structure, through which only pure water flows.
[1] Rott, Ulrich: Gutachterliche Stellungnahme zur Wirkungsweise der ”FERMANOX-Wasseraufbereitung” unter besonderer Berücksichtigung umweltrelevanter Auswirkungen. Universität Stuttgart, Institut für Siedlungswasserbau, Wassergüte- und Abfallwirtschaft, 1994
[2] Rott, Ulrich: Gutachterliche Stellungnahme zur Aufbereitungsleistung der ”FERMANOX-Wasseraufbereitung”. Universität Stuttgart, Institut für Siedlungswasserbau, Wassergüte- und Abfallwirtschaft, 1995
[3] Rott, Ulrich; Meyerhoff, Ralf ; Bauer, Tarja: In situ-Aufbereitung von Grundwasser mit erhöhten Eisen-, Mangan- und Arsengehalten. In: gwf – Wasser/Abwasser 137 (1996.) Nr. 7, S. 358-363
The basic requirement for the use of FERMANOX® systems for underground iron and manganese removal is vertical or horizontal filter wells in loose rock (sand or gravel), because the actual processing requires, above all, the large surface area of a porous aquifer. The usual technical rules apply to the well construction (especially DVGW W 113, W 123 and DIN 4924). In addition, it is particularly important to ensure that no vertical flows occur in the well structure.
There are application limits with regard to the quality of the raw water. Demanganization in particular requires a minimum pH value, which is lower than with above-ground processes due to the high efficiency of the process. In addition, the treatment effort increases significantly with higher ammonium or methane concentrations in the groundwater. There are no limits for iron or manganese concentrations in the raw water (contrary to various publications, see also [1]). With FERMANOX®, even extreme groundwater can be treated to drinking water level.

In order to assess the applicability of the process, offer a suitable water treatment plant and ensure the usual FERMANOX® guarantee (below the drinking water limit values for iron, manganese and ammonium), we therefore require the following documents:
- Raw water analyses of all wells
- Layer and development lists of all wells (→ DIN 4023 and 4943)
- Site plan
- Information on pumping capacity and concept
- Records of well development (if available, e.g. records of de-sanding and power pumping tests)
- Possibly hydrogeological reports
If necessary, preliminary tests are useful, for which we can offer test systems.
[1] Groth, Peter ; Czekalla, Christian ; Dannöhl, Rainer ; Kölle, Walter ; Ließfeld, Rainer ; Meyerhoff, Ralf ; Olthoff, Reinhold ; Rott, Ulrich ; Wiegleb, Klaus: Unterirdische Enteisenung und Entmanganung – aktualisierter Statusbericht. In: gwf – Wasser/Abwasser (Sonderdruck) 138 (1997) Nr. 4, S. 182-187
Lifespan of boreholes
The actual volume of oxidation products is usually significantly overestimated, while the size of the reaction space in the aquifer is underestimated:
A case study
| Assumptions: | |
|---|---|
| Water withdrawal in 30 years: | 10.950.000 m³ |
| Water extraction: | 1.000 m³/day |
| Iron content: | 5 mg/l |
| Operating time: | 30 years |
The amount of water assumed in the example contains 54.75 t of iron, which – assumed as an iron block – takes up a volume of 6.97 m³. This amount of iron results in 87.6 t of crystalline iron oxide hydrate with a volume of only 21.4 m³ through underground water treatment.
The real pore volume of a naturally grown aquifer (fine sand to coarse gravel) is 36% to 42%. If only 36% is assumed, the entire iron oxide hydrate from 30 years of water extraction could be accommodated in just under 60 m³ of sand. With an average reprocessability with a yield factor of KE = 5, however, the above calculation achieves around 850 m³ of soil volume from the oxygen input. This means that after the first 30 years of operation, significantly more than 90% of the pore volume in the aquifer is still available.
In addition, the deposition of oxides takes place primarily in the hydraulically irrelevant dead-end pores. And finally, the reaction space increases to the extent that active pore volume is closed by deposition, because the amount of oxygen-enriched water returned remains the same.
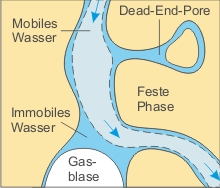
Section of a porous aquifer after [4]
These results are confirmed by numerous publications in the specialist literature (e.g. [2]) and by our own experience. The Rheindahlen waterworks of NiederrheinWasser GmbH has been carrying out underground iron removal for over 30 years without any negative hydraulic effects being detected [3]. The first FERMANOX® system was put into operation in 1983 with an iron concentration of 6.7 mg/l. The system has been running smoothly to this day, although the borehole is now around 60 years old and has never been regenerated.
[1] Groth, Peter ; Czekalla, Christian ; Dannöhl, Rainer ; Kölle, Walter ; Ließfeld, Rainer ; Meyerhoff, Ralf ; Olthoff, Reinhold ; Rott, Ulrich ; Wiegleb, Klaus: Unterirdische Enteisenung und Entmanganung – aktualisierter Statusbericht. In: gwf – Wasser/Abwasser (Sonderdruck) 138 (1997) Nr. 4, S. 182-187
[2] Friedle, Matthias ; Rott, Ulrich: Grundlagen und Anwendungen von Verfahren zur subterrestrischen Aufbereitung von Grundwasser. Handbuch Wasserversorgungs- und Abwassertechnik. Vulkan-Verlag, 1998, S. 79-107
[3] Ewert, Thomas; Wisotzky, Frank; Schindler, Roland; Schumacher, Detlef; Rott, Ulrich: Erfahrungen mit der unterirdischen Enteisenung an den Wasserwerken der NiederrheinWasser GmbH. In: gwf – Wasser/Abwasser 152 (2011) Nr. 2, S. 170-175[4] Schulte-Ebbert et al.: Verhalten von anorganischen Spurenstoffen bei wechselnden Redoxverhältnissen im Grundwasser. Veröffentl. Des Instituts für Wasserforschung GmbH Dortmund und der Dortmunder Stadtwerke AG, Nr. 32; 1991
FERMANOX WV & WV Professional for waterworks
Water treatment plant for underground iron and manganese removal with FERMANOX
Area of application
The FERMANOX® stainless steel systems of type WV & WV professional are designed for industrial performance. They are operated alternately with two or more boreholes and are used in different performance classes and designs depending on the respective requirements.
Water treatment performance
Depending on the raw water quality, systems of type WV professional have a treatment capacity of 100 to a maximum of 8,000 m³/day per system. To increase performance, several systems can be connected in parallel.

FERMANOX®-System type WV professional
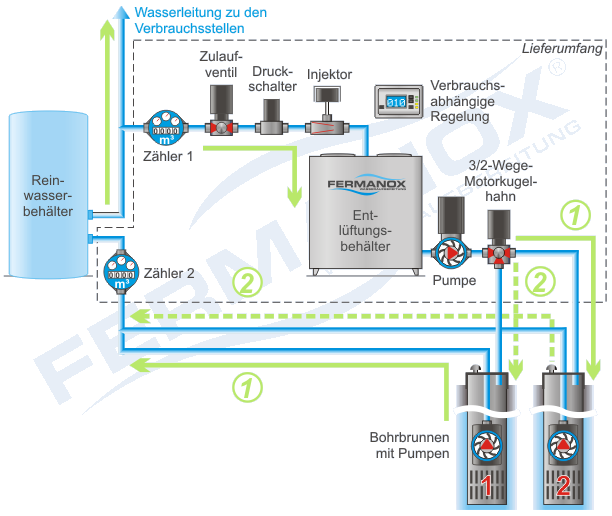
Flow diagram for a water supply with FERMANOX® system type WV Professional
functionality
The FERMANOX® stainless steel systems of type WV professional work with at least two drilled wells, which are operated alternately as infiltration wells and as production wells.
A small part of the water from the well in production is enriched with oxygen from the air and passed through a degassing tank and then re-filtered into the aquifer via the infiltration well.
After a certain production and infiltration volume has been passed through, the well functions are switched. Treated water is then pumped from the previous infiltration well, while oxygen-rich water is infiltrated via another well.
Consumption-dependent regulation
In FERMANOX® systems of the type WV professional, the amount of water used and the amount returned for treatment are continuously monitored. The consumption-dependent control ensures that the correct ratio of pumping and oxygen enrichment is always maintained for each well. At the same time, the next enrichment only takes place after a corresponding amount of water has been used.
If there is a deviation from the target data, an alarm is automatically triggered and the cause of the error is displayed.

Special injector of the FERMANOX® system type WV professional
Applications with extremely fluctuating water consumption
Even for special applications with extremely low water consumption over a longer period of time, Winkelnkemper GmbH can guarantee the permanent functionality of the system by programming the system accordingly. At the same time, this control achieves optimal conditions with the lowest possible energy consumption.
Monitoring and remote maintenance
The consumption-dependent controlled system also monitors itself and gives an alarm if there are deviations from normal operation.
It is possible to log and read out the operating data. A PC can also be connected to the control interface for this purpose. Alternatively, remote reading and parameterization or control center connection are possible.

Consumption-dependent control with readout software
FERMANOX®-interpretation
The right ratio of extraction and enrichment is crucial for the success of the process. Specifically, for each well, the return volume must be adapted to the daily water requirement and the raw water quality of the groundwater. We therefore offer 3 different standard sizes with different equipment under the type designation WV. The selection of the suitable system can only be made after we have carried out an individual design.
If these parameters deviate significantly from the original starting data in the future (e.g. due to an increase in water requirements), an existing system must be redesigned by the manufacturer in order to avoid calcification of the wells.
FERMANOX®-Test facilities
Area of application
For large projects and in cases where we cannot guarantee the functionality of the process from the outset due to unfavorable raw water quality or soil conditions, a trial operation is recommended. For this purpose, we offer FERMANOX® WV professional test systems and accessories for rent, as well as services related to test support.

FERMANOX®-Test facility in container
A crucial step in the construction or modernization of a waterworks is the process engineering planning of the water treatment. When choosing between above-ground and underground iron and manganese removal, it is worth comparing both alternatives based on efficiency and cost-effectiveness.
If you compare underground iron and manganese removal with an open gravel filter without additives (i.e. without hardening or addition of auxiliary materials, etc.), the following qualitative picture emerges:
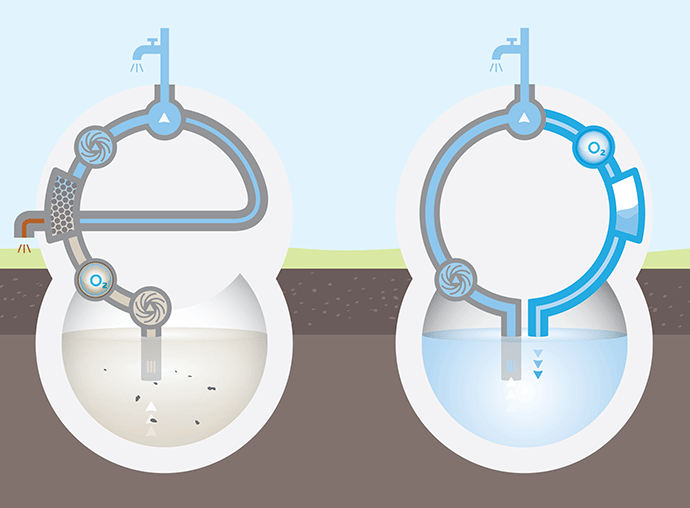
Concept comparison FERMANOX compared to the gravel filter process
The FERMANOX® water treatment process is probably the most efficient on the market
To understand the significantly higher efficiency of underground iron and manganese removal compared to above-ground rapid filters, a comparison of the reaction chambers is helpful.
With an effective pore volume of around 25% in a loose rock aquifer, infiltration creates a reaction chamber that has a volume of around 4 times the infiltration volume – many times larger than with conventional rapid filters. In this large chamber, the water is pumped to the well. Infiltration takes place in the opposite direction from the well into the aquifer. In contrast to conventional filters, the entire reaction chamber is thus active for the treatment, and the processes of oxidation and adsorption are largely separated, which is advantageous. Since the pumped volume always exceeds the infiltration volume, there is no change in the equilibrium prevailing in the aquifer beyond the reaction chamber.
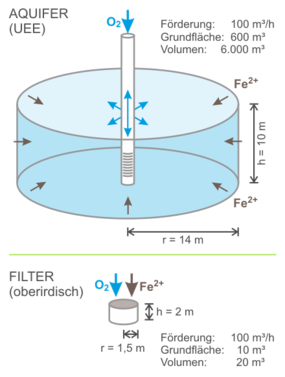
Comparison of reaction space sizes in the aquifer and in the above-ground filter after [1]
The large reaction space or the huge surface area active for adsorption and reaction, a long reaction time and the more favourable countercurrent principle in this reaction space result in an efficiency that is practically unattainable with above-ground processes. As a result, UEE achieves significantly higher treatment performance with lower oxygen requirements (and lower energy consumption). Even groundwater with extremely high iron and manganese concentrations can be treated with FERMANOX® to a concentration well below the limits of the Drinking Water Ordinance.
Underground water treatment offers high economic efficiency
„Economic requirements, in particular the need to use available resources sparingly in terms of space requirements, construction and operating costs in waterworks, require constant improvements in the state of the art.“ [1]
The DVGW confirms that underground iron and manganese removal has lower investment and operating costs compared to conventional filter systems and attributes this primarily to the fact that only a few above-ground system components are required.[2]
A more detailed cost-benefit comparison shows other advantages of underground iron and manganese removal in addition to the significantly lower costs for the technical equipment.

- Due to the small construction volume of underground iron and manganese removal, the water treatment enclosure can be much smaller than with above-ground systems. This not only saves investment costs, but also energy costs for heating and drying the operating rooms.
- The higher energy efficiency alone ensures a cost advantage with the UEE that exceeds the investment costs over the service life (which is long for waterworks).
- There is no backwashing, no filter changes, no waste water or waste with the UEE. A fully automated and maintenance-free system such as the FERMANOX® type WV professional reduces operating costs to a minimum.
- Due to the lower operating costs compared to above-ground filters alone, modernization with a FERMANOX® system almost always pays for itself within a short time.
- When replacing old systems, a seamless transition without interruption to the drinking water supply is achieved by temporarily operating both systems.
The FERMANOX® water treatment process guarantees iron, manganese and ammonium-free water – directly from the well.

Reference projects
Type: WV 80/1/40 P Nr. 2005
Capacity: 380m³/day
In operation since 2017
Type: WV 100/1/100 P in a container
Capacity: 1000m³/day
In operation since 2012
Type: WV 80/1/40
Capacity: 350m³/day
In operation since 2004
Type: WV 100111200 P – 3B
Capacity: 600m³/day
In operation since 2017







