



Water – Our vitality fountain | Drinking water treatment for private households
Water is our most important food.
Almost every process in the body depends on water. It transports nutrients and oxygen to the cells via the bloodstream. If you drink enough water regularly, your cells are better supplied and you therefore feel more efficient, more alert and more concentrated, and therefore more vital. Drinking water is therefore extremely important for good health – with FERMANOX drinking water treatment for private households, you get drinking water directly.

From your well – groundwater directly into your glass!
Groundwater – Naturally healthy
In Germany, drinking water is mostly obtained from groundwater and is therefore part of the natural water cycle.
As the water slowly seeps through the soil layers, it is naturally purified of many undesirable substances. At the same time, it dissolves valuable minerals and trace elements and absorbs them – depending on the rock structure – in different concentrations and compositions.
Groundwater is therefore a reflection of its region, which gives the water its individual character and taste on its way into the soil.
How much water do you get on average for one cent?
According to current statistics, the average per capita consumption of drinking water in Germany is 121 litres per inhabitant per day for private consumption.

Mineral water
A single bottle of mineral water in Germany costs an average of 50 cents per liter, i.e. for 1 cent you only get 20 ml of mineral water. (= 1 shot glass full of water).

Public water
A cubic meter of drinking water (1000 liters) costs an average of 1.65 euros in Germany. One liter of water therefore costs 0.00165 euros or 0.165 cents, i.e. for 1 cent you get about 6 liters of drinking water from the public supply. (= 1/2 bucket of water).

Own water
The cost of your own water is primarily made up of the energy costs for pumping the water. These amount to around 5 cents per cubic meter (= 1000 liters); i.e. for 1 cent you get around 200 liters of water. (= 2 bathtubs).
Stiftung Warentest (test issue 07/2012) also states that drinking water is “dirt cheap” and of high quality compared to mineral water and also often contains more minerals.
Groundwater – the clear winner in the ecological balance of drinking water

Consuming mineral water requires around 1,000 times as much energy as drinking water. The difference is greater the further the mineral water is transported – from the bottling site to the consumer.
The most positive ecological balance is for using your own groundwater as drinking water. Long transport routes are eliminated here, as the water from your own property is used. Energy costs are therefore only incurred for pumping the water from the well.
In addition, no packaging material is required, as is the case with mineral water. Our own water is also always available in a cool state.
Problems of groundwater use
Groundwater is formed from rainwater that seeps into the soil. As it seeps through the soil layers, the water is microbiologically purified, but at the same time it becomes increasingly oxygen-poor.
In the deep groundwater-bearing soil layers, the groundwater usually no longer contains any oxygen. Due to the lack of oxygen, iron (Fe2+) and manganese (Mn2+) dissolve in increased concentrations. Ammonium (NH4+) can also be formed during biological decomposition processes under a lack of oxygen. In dissolved form, these substances cannot be filtered out.

Water with high iron (Fe2+), manganese (Mn2+) and ammonium (NH4+) contents requires treatment before it can be used as drinking water in accordance with the Drinking Water Ordinance (TrinkwV)[1]. In addition, water containing iron and manganese causes brown and black deposits and discoloration.
Drinking water quality – directly from the well, with FERMANOX® drinking water treatment.

Drinking water quality is characterized above all by its high sensory quality. Drinking water should be clear, pure and appetizing.
Water containing iron is cloudy. It turns brown, looks unappetizing and tastes bad.
For the so-called “indicator parameters” iron, manganese and ammonium, the Drinking Water Ordinance also specifies clear limits on how high the maximum concentrations in drinking water may be.
Healthy water | Limit values according to the Drinking Water Ordinance
Iron and manganese are the two most common heavy metals in the earth’s crust, often occur together in minerals and are often both present in high concentrations in groundwater.
The geogenic concentrations are usually well below the range that is relevant to the health of an adult. The Drinking Water Ordinance sets significantly lower limit values for sensory and technical reasons[1].
The Drinking Water Ordinance was amended in November 2011. Since then, the low limit values of the Drinking Water Ordinance have been binding for all users. There are no longer any exceptions for small suppliers either. FERMANOX® guarantees compliance with the drinking water limit values not only for iron, but also for manganese and ammonium. In addition, FERMANOX® also guarantees that the drinking water limit values are not exceeded for other oxidizable ingredients such as nitrite, arsenic, hydrogen sulphide and methane, although these occur less frequently.
[1] TrinkwV: Trinkwasserverordnung in der Fassung der Bekanntmachung vom 2. August 2013 (BGBl. I S. 2977)
Extract from the Drinking Water Ordinance[2]
§ 7 Indicator parameters and appendix 3
| Parameter | limit |
|---|---|
| Ammonium: | 0,5 mg/l |
| Iron: | 0,2 mg/l |
| Manganese: | 0,05 mg/l |
These low limit values are sensible because iron concentrations > 0.5 mg/l result in directly perceptible changes (brown discoloration of the water when exposed to air, change in the taste of the water). From 0.2 mg/l, discoloration and deposits can also occur on surfaces that come into contact with water, which limits both usability (from the customer’s perspective) and technical operational safety (from the supplier’s perspective → ochre).
In addition, deposits of iron oxide hydrates in pipes can form the basis for undesirable microorganisms[4]. To avoid this, the drinking water supplied by the waterworks should not contain more than 0.05 mg/l of iron[5]. The DVGW even recommends iron concentrations ≤0.02 mg/l and manganese concentrations ≤0.01 mg/l.[6] In reduced groundwater, in addition to iron and manganese, ammonium is often also found in increased concentrations. A limit value is also set for this in the Drinking Water Ordinance for general hygiene reasons. In general, compliance with these limits is mandatory for the supply of drinking water, but most water suppliers place significantly higher demands on their own drinking water.
This also corresponds to the minimization requirement required in the Drinking Water Ordinance and DIN 2000: “Concentrations of chemical substances that can contaminate drinking water or adversely affect its quality should be kept as low as is possible with reasonable effort in accordance with the generally accepted rules of technology.”[2]
This implies that water suppliers must choose a treatment technology that is as efficient and economical as possible. In the case of increased concentrations of iron, manganese or ammonium, underground iron removal and manganese removal (UEE) is recommended according to DVGW worksheet W 223[6].
[1] DIN 2000: Zentrale Trinkwasserversorgung – Leitsätze und Anforderungen an Trinkwasser – Planung, Bau, Betrieb und Instandhaltung der Versorgungsanlagen; Technische Regel des DVGW
[2]TrinkwV 2001: Trinkwasserverordnung in der Fassung der Bekanntmachung vom 2. August 2013 (BGBl. I S. 2977)
[3] Richtlinie 98/83/EG des Rates: über die Qualität von Wasser für den menschlichen Gebrauch.
[4] Höll, Karl: Wasser. Berlin [u.a.]: 8., völlig neu bearb. Aufl. Aufl. de Gruyter, 2002.
[5] Grohmann, Andreas ; Hässelbarth, U. ; Schwerdtfeger, W.: Die Trinkwasserverordnung. Berlin: 4., neu bearb. Aufl. Aufl. E. Schmidt, 2003.
[6] DVGW: Arbeitsblatt W 223-1. 2005.
Various processes between chemical and biological components of the groundwater and the composition of the soil lead to well aging or calcification in the course of well operation.
This refers to the reduction in well performance, primarily due to the deposition of reaction products in the well structure or in the adjacent rock and the resulting reduction in the cavity volume.[1]

Partially ochered wound wire filter
As soon as the water inlet areas into the well structure are reduced, the flow rate drops or the lowering of the operating water level increases while the withdrawal volume remains the same.
The main causes are:
- chemical and biological ochre formation
- sintering
- slime formation
- silting
While silting can result from errors in well construction, the other causes depend primarily on the quality of the raw water and the operation of the well.[2]
Clogging as the most common form of well aging
„The oxidation of dissolved, divalent iron and manganese and the resulting precipitation of insoluble iron(III) or manganese(IV) compounds is the most common cause of well aging.“ [3]
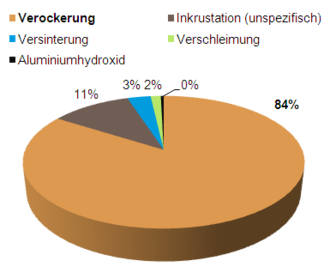
Ageing processes of wells in Germany according to a DVGW survey[5]
A 2009 DVGW survey found that two thirds of all waterworks operators experience well aging, which is usually detected by loss of yield or directly by camera inspection. This aging requires the regeneration and rehabilitation of wells, which most waterworks operators carry out at intervals of 1 to 5 years.[1]
A previous DVGW survey showed that the cause is almost always iron oxide. Over 80% of this is iron oxide, the rest is manganese oxide.[1]
Chemical and biological ochre
Chemical ochre is caused by the oxidation of divalent species when dissolved iron (Fe2+) or manganese ions (Mn2+) come into contact with dissolved oxygen. The reaction rate depends strongly on the pH value, but increases significantly due to the autocatalytic effect of oxides that have already formed. Since the oxidation of iron and manganese requires different redox potentials, either rust-brown iron ochre or black manganese ochre occurs, but rarely both. Where both species are present in the groundwater, only the iron is oxidized initially.

At lower redox potentials, biological ochre deposits occur in the majority of cases. The oxidation is catalyzed by sessile bacteria (e.g. by “iron bacteria”: Gallionella ferruginea, Leptothrix ochracea and discophorus). These bacteria require a regular supply of nutrients from water flowing past. They are harmless to drinking water hygiene, but in addition to the biomass they form large amounts of oxides (as well as hydroxides and oxide hydrates).
The deposits are generally subject to an aging process in which initially highly water-containing amorphous oxides change into denser crystalline forms, which are then more stable and difficult to dissolve. Iron-related incrustations usually occur in the form of ferrihydrite (Fe5HO8 x 4 H2O) and goethite (FeOOH)..[1]
Conclusion:
In summary, the ochre formation of wells is based on the following factors:
- Dissolved iron or manganese ions (according to specialist literature at iron concentrations > 0.2 mg/l[8])
- A positive redox potential / dissolved oxygen
- A pH value > 5
- An increased flow rate
- Iron and manganese-storing bacteria

Clogging of an underwater pump impeller
These factors are often present in well structures. If the performance drops by 10% to 20% due to ochre, an advanced stage of aging has already been reached and “regenerating it requires a high level of technical effort and therefore high costs.”[9]
As a preventive or remedial measure, attempts are often made to positively influence the oxygen level and the entry speed in the filter area through structural measures. But the most efficient method of preventing ochre is the use of underground iron and manganese removal.
This efficiently removes iron and manganese from the water before it enters the well structure.
[1] Houben, Georg ; Treskatis, Christoph: Regenerierung und Sanierung von Brunnen. München: Oldenbourg Industrieverl, 2003.
[2] Tholen, Michael: Arbeitshilfen für den Brunnenbauer. 2. Aufl. 2012.
[3] Wiacek, Hella: Brunnenmonitoring zur optimalen Brunnennutzung und -pflege. Johannes Gutenberg-Universität Johannes Gutenberg-Universität in Mainz, Fachbereich Chemie, Pharmazie und Geowissenschaften, 2005
[4] Orlikowski, D ; Dauchy, L ; Schwarzmüller, H: Ergebnisse der bundesweiten DVGW-Umfrage zur Instandhaltung von Brunnen. 2009.
[5] Houben, Georg ; Treskatis, Christoph: Regenerierung und Sanierung von Brunnen. München: Oldenbourg Industrieverl, 2003.
[6] Niehues, B.: DVGW-Umfrage ”Brunnenregenerierung”. 1999.
[7] Houben, Georg ; Treskatis, Christoph: Regenerierung und Sanierung von Brunnen. München: Oldenbourg Industrieverl, 2003.
[8] Mutschmann, Johann ; Stimmelmayr, Fritz: Taschenbuch der Wasserversorgung. Wiesbaden: 14. Aufl. Friedr. Vieweg & Sohn Verlag, 2007.
[9] DVGW: Arbeitsblatt W 130, 2007.
The solution for process water treatment: Underground iron and manganese removal (UEE)
The underground iron and manganese removal of groundwater (process water treatment) (UEE) with FERMANOX® activates a natural treatment process in the aquifer before extraction. With little effort, iron and manganese concentrations at drinking water level can be generated there. This effectively prevents all discoloration and ochre formation. At the same time, the process is particularly sustainable because
- no filter material is required,
- no waste water or waste is produced,
- energy requirements are particularly low and
- the service life of the boreholes is increased.

FERMANOX®-Plant for underground iron and manganese removal
The process: Underground iron and manganese removal with FERMANOX
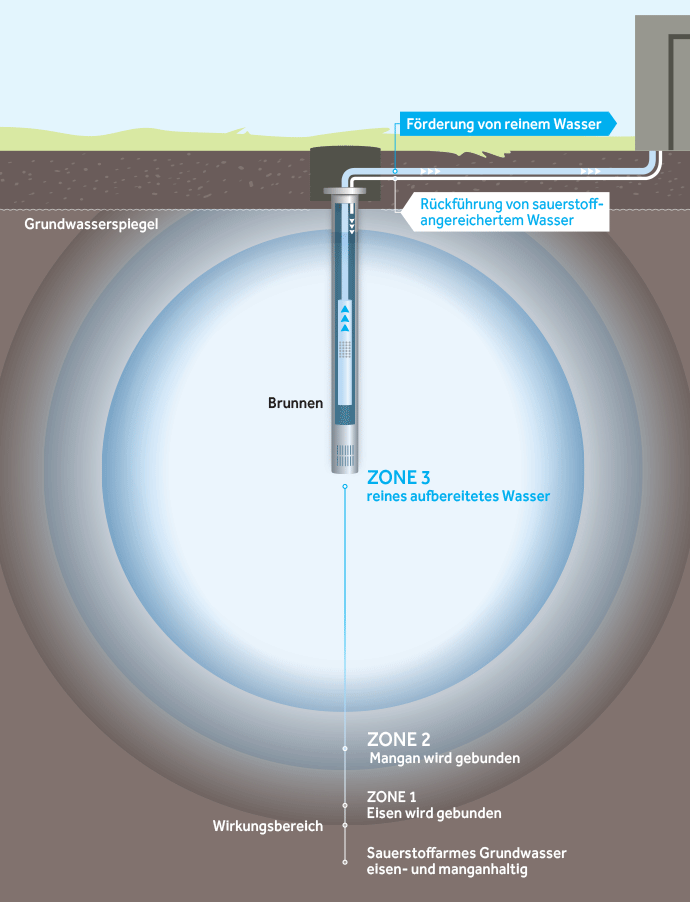
Oxidation zones in the aquifer when using an UWE
The basic idea behind underground deferrification and demanganization is the introduction of water enriched with atmospheric oxygen into the aquifer. Even small amounts of oxygen activate an effective water treatment process there, because a reaction space with an increased redox potential is created around the well.
The substances that were dissolved in the aquifer due to reduced conditions are returned to the solid state by oxidation and are then permanently fixed again in the same aquifer at another location. This achieves efficient deferrification and demanganization of the groundwater and also nitrification of ammonium, a reduction in easily oxidizable organic substances and a reduction in arsenic.
Continuous underground water treatment is based on the operation of at least two wells that work alternately as extraction or infiltration wells. The size of the reaction space around each well is determined by the amount of infiltration and the proportion of active pore volume in the aquifer. It requires an individual design for each well, which is based primarily on the raw water quality and the required treatment capacity.
History
The underground iron and manganese removal of groundwater has been used on a large scale since the 1970s, and since the 1980s in a variety of small and medium-sized plants for drinking and industrial water treatment, primarily in Germany, the Netherlands and Scandinavia, in various designs. Winkelnkemper GmbH has been manufacturing FERMANOX® water treatment systems since 1984 and is now the market leader in the field of underground iron and manganese removal, with over 10,000 compact systems installed.
The basic requirement for the use of FERMANOX® systems for underground iron and manganese removal is vertical or horizontal filter wells in loose rock (sand or gravel), because the actual processing requires the large surface area of a porous aquifer. The usual technical rules apply to the well construction (especially DVGW W 113, W 123 and DIN 4924). In addition, it is particularly important to ensure that no vertical flows occur in the well structure.
There are application limits with regard to the quality of the raw water. Demanganization in particular requires a minimum pH value, which is lower than with above-ground processes due to the high efficiency of the process. In addition, the treatment effort increases significantly with higher ammonium or methane concentrations in the groundwater. There are no limits for iron or manganese concentrations in the raw water (contrary to various publications, see also [1]). With FERMANOX®, even extreme groundwater can be treated to drinking water level.

In order to assess the applicability of the process, offer a suitable water treatment plant and ensure the usual FERMANOX® guarantee (below the Drinking Water Ordinance limit values for iron, manganese and ammonium), we therefore require the following documents:
- Raw water analyses of all wells
- Layer and development lists of all wells (→ DIN 4023 and 4943)
- Site plan
- Information on pumping capacity and concept
- Records of well development (if available, e.g. records of de-sanding and power pumping tests)
Possibly hydrogeological reports If necessary, preliminary tests are useful, for which we can offer test systems.
[1] Groth, Peter ; Czekalla, Christian ; Dannöhl, Rainer ; Kölle, Walter ; Ließfeld, Rainer ; Meyerhoff, Ralf ; Olthoff, Reinhold ; Rott, Ulrich ; Wiegleb, Klaus: Unterirdische Enteisenung und Entmanganung – aktualisierter Statusbericht. In: gwf – Wasser/Abwasser (Sonderdruck) 138 (1997) Nr. 4, S. 182-187
The FERMANOX® water treatment process guarantees iron, manganese and ammonium-free water – directly from the well.

Advantages of FERMANOX® water treatment
Healthy, delicious water
This is important because since 2011 there have been no exceptions for small suppliers in the case of (geogenically caused) increased concentrations.

No discoloration
Water treated with FERMANOX® is free of iron and manganese and therefore does not leave any brown or black discoloration when used. This keeps laundry, sinks, showers, bathtubs and tiles clean.

No deposits
Since underground treatment prevents iron and manganese deposits (calcification) on wells, pumps and pipes, cleaning or regeneration is no longer necessary.
The FERMANOX® system significantly extends the service life of your entire water supply system, as only pure, treated water flows through wells, pumps and pipes.

Submersible pumps after 5 years of operation with FERMANOX® (above) and a conventional gravel filter (below)
Maximum efficiency – minimum energy consumption
In contrast to conventional methods, the FERMANOX® system only requires a small amount of oxygen-rich water, which is fed into the aquifers for treatment, since the natural reaction zone in the area surrounding the well is many times larger than the filter volume of above-ground filters.
As a result, the energy requirement for the entire water supply is significantly lower than with conventional water treatment.
No maintenance – just checks!
Since the water treatment in the FERMANOX® process takes place in the aquifer itself, no regular maintenance is required, just an inspection.
In small FERMANOX® water treatment systems, the strainer in front of the system must be checked once a year to ensure that the oxygen enrichment performance does not drop due to blockages. Large systems are completely maintenance-free.
No filters, no backwash
In contrast to conventional methods, no above-ground filters are necessary. The water is naturally treated before it is pumped and used – without chemicals or replacement material.
Annoying and expensive replacement and disposal of filter material as well as backwashing of the filters are no longer necessary.
[1] The legal basis is the Drinking Water Ordinance in the version published on 28 November 2011 (BGBl. I page 2370), which was amended by Article 2 paragraph 19 of the Act of 22 December 2011 (BGBl. I p. 3044).
FERMANOX®-water treatment systems for private households
The requirements for a water treatment plant for industrial water vary depending on the water requirement, water quality and control requirements in the specific application. Depending on the size of the company and the quality of the raw water, the following FERMANOX® water treatment plant types are available:
| FERMANOX®-type | Water supply | Area of application | Control/regulation | Possibility of monitoring |
|---|---|---|---|---|
| Type BV | with 1 abstraction well | for small capacities; up to 15 m³/day | consumption-dependent | yes |
| Type BZ | with 1 abstraction well | for small capacities; up to 10 m³/day | time-dependent | no |
Versions of the different basic types
Behind each of the FERMANOX® basic types listed above there are a variety of systems of different sizes and equipment (e.g. type BV 30 P). The suitable system can only be selected after an individual design (depending on the water requirement and the water quality of the groundwater) by Winkelnkemper GmbH.

Installation example FERMANOX®-BV | 1-well system | Small water requirement
Installation example water treatment plant FERMANOX BV (illustration)
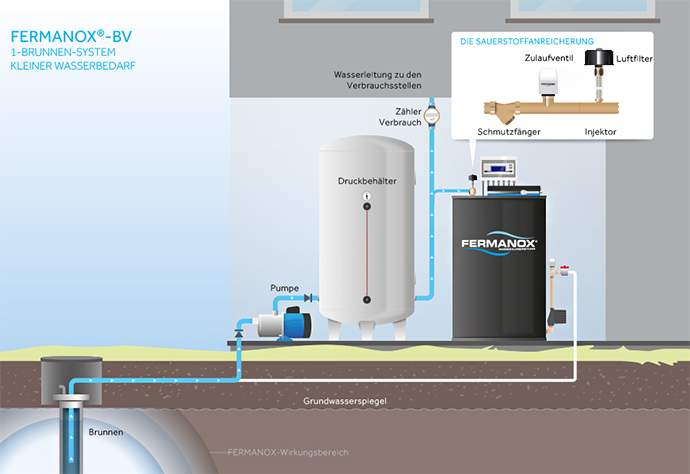
Illustration FERMANOX®-BV | 1-well system | Small water requirement
A crucial step in the construction or modernization of houses is the process engineering planning of the water treatment. When choosing between above-ground and underground iron and manganese removal, it is worth comparing both alternatives based on efficiency and cost-effectiveness.
If you compare underground iron and manganese removal with an open gravel filter without additives (i.e. without hardening or addition of auxiliary materials, etc.), the following qualitative picture emerges:
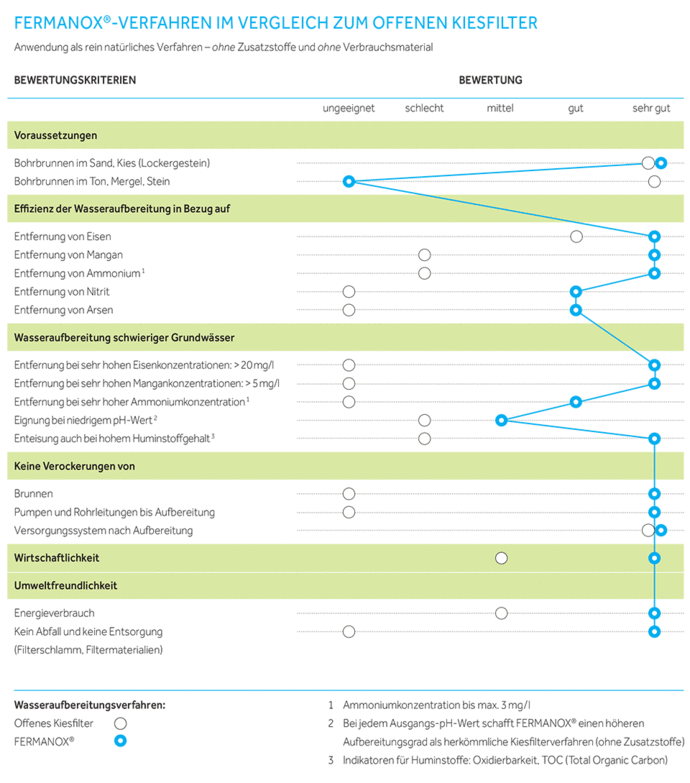
Concept comparison FERMANOX compared to the gravel filter process
The FERMANOX® water treatment process is probably the most efficient on the market
To understand the significantly higher efficiency of underground iron and manganese removal compared to above-ground rapid filters, a comparison of the reaction chambers is helpful.
With an effective pore volume of around 25% in a loose rock aquifer, infiltration creates a reaction chamber that has a volume of around 4 times the infiltration volume – many times larger than with conventional rapid filters. In this large chamber, the water is pumped to the well. Infiltration takes place in the opposite direction from the well into the aquifer. In contrast to conventional filters, the entire reaction chamber is thus active for the treatment, and the processes of oxidation and adsorption are largely separated, which is advantageous. Since the pumped volume always exceeds the infiltration volume, there is no change in the equilibrium prevailing in the aquifer beyond the reaction chamber.
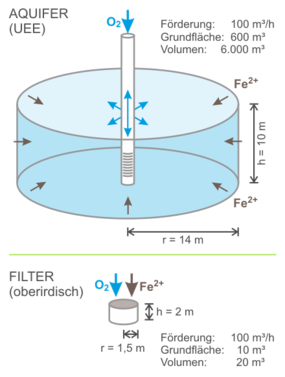
Comparison of reaction space sizes in the aquifer and in the above-ground filter after [1]
The large reaction space or the huge surface area active for adsorption and reaction, a long reaction time and the more favourable countercurrent principle in this reaction space result in an efficiency that is practically unattainable with above-ground processes. As a result, UEE achieves significantly higher treatment performance with lower oxygen requirements (and lower energy consumption). Even groundwater with extremely high iron and manganese concentrations can be treated with FERMANOX® to a concentration well below the limits of the Drinking Water Ordinance.







